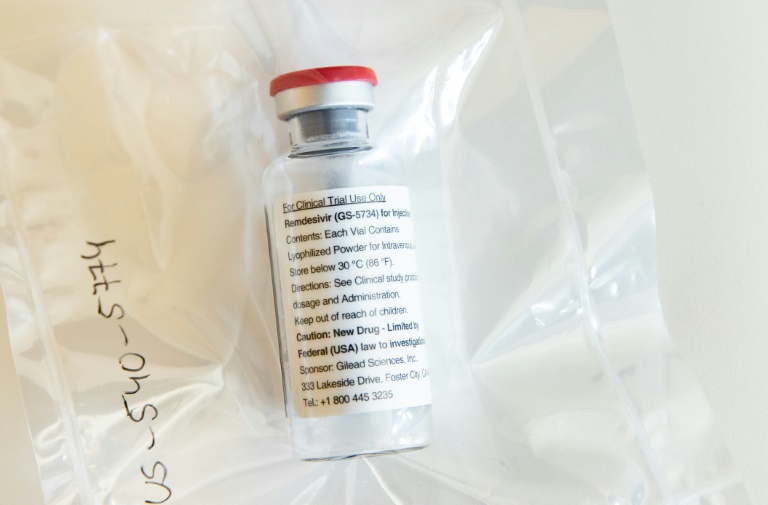
The US Food and Drug Administration (FDA) approved on Friday an experimental antiviral drug, Remdesivir, for emergency use in COVID-19 patients. The drug can now be given to patients who are hospitalised with severe symptoms and need supplemental oxygen therapy or a ventilator. Emergency use authorisation means that unapproved drugs that might help treat a life-threatening disease can be used when no approved alternatives are available.
Earlier two clinical trials had shown that Remdesivir was effective in shortening the disease duration in hospitalised patients.
Source: Samaa

Inflics provides it readers the information that they need in concise and short articles, making information and news more accessible to everyone.